Pancreatic cancer is the 10th most common cancer, affecting 10,500 people in the UK (1) and 64,050 in the United States each year. It is the fourth leading cause of cancer death in both men and women (2).
Unfortunately, as many as 80% of people with pancreatic cancer are diagnosed with advanced-stage disease, as it typically presents with little or no symptoms (2). Once in the advanced stages, it becomes very difficult to treat successfully. On average, 25% of people will survive 1 year after diagnosis, and this drops to 5% reaching 5 and 10-year survival.
Treatment options
Surgical intervention is currently the only treatment option that provides a feasible treatment solution for survival; however with advanced-stage disease the tumor is typically already unresectable, with 50% of patients having distant metastases present at diagnosis (3). Operative treatment is possible in about 15-20% of patients with newly diagnosed pancreatic cancer. Regional recurrence rate is as high as 85% after surgical treatment (4).
A multi-modality approach to treatment is therefore needed to improve prognosis however, statistics remain low.
Chemotherapy will often be given post-surgery or in combination with a radiation treatment, which could be photons, protons or carbon ions. Conventionally the chemotherapy regime will be gemcitabine-based or follow the FOLFIRINOX regime (4).
Radiation therapies
Radiation therapies have long shown promise in the treatment of this disease when considering the high risk of local-regional recurrence associated with this cancer type (5).
The pancreas is surrounded by radio-sensitive organs such as the stomach, duodenal loop, large and small intestines, liver, kidney and spinal cord (4). This makes treating with photons difficult often resulting in dose restrictions to reduce toxicities or failure to complete treatment altogether due to concerns regarding gastrointestinal perforation or ulceration which can be fatal (5). Some success has been seen with the development of stereotactic radiotherapy. IMRT has also reduced toxicities, as well as the development of simultaneous imaging techniques, which allow for real-time imaging of the tumor itself (6). The development of the MR-linac has also improved the outcomes of radiation treatments for this disease. Stereotactic MR-guided adaptive radiotherapy allows for continuous real-time imaging of internal structures with the advantage of better soft tissue visualization than computed tomography (CT). Real-time tumor position tracking has allowed for dose escalation with sparing to surrounding organs at risk and with the advantage of radiation-free repeat scans. The results from studies into the use of the MRI-LINAC showed minimal toxicity during and after treatment even in previously resected or irradiated patients (7).
Carbon ion therapy
Carbon ion therapy has been gaining traction in the radiation therapy world due to its many advantageous properties. Properties that have the potential to make a positive impact on pancreatic cancer statistics.
Carbon ion therapy, much like proton therapy, is highly conformal and precise and it has a favorable energy distribution at depth seen with its unique Bragg Peak. With carbon ions there is relatively low energy deposition at the entrance of the patient then a very steep gradient with maximum energy of the particle being deposited at the tumor (8), followed by an immediate drop off in energy which means the surrounding healthy tissue receives very little dose. A consideration when comparing carbon ions with protons is the deposition of energy distally from nuclear fragmentation creating a dose tail which is seen with carbon ions, the impact of this is typically compensated with the use of multiple beam angles (9).
Carbon ions also differ from protons favorably in their mass. Carbon ions are 12 times heavier than a proton (10). They have a higher Linear Energy Transfer (LET) than photons and protons, which leads to a higher Relative Biological Effectiveness (RBE). This results in damage caused by the carbon ions being clustered within the DNA and does not require the generation of free radicals to cause this damage, as with photon treatment (8, 23). This type of direct damage to the DNA strands overwhelms the cellular repair system. Due to these biological characteristics, carbon ions are deemed to be the most effective in treating hypoxic radioresistant tumors (11), with pancreatic cancer falling into this category.
A 2016 study (12) evaluated the feasibility of planning and delivering carbon ion therapy for pancreatic cancer and reported local tumor control rates of 92% at 1 year and 83% at 2 years. Another study published in 2018 (13) reported a median overall survival of 21.5 months after carbon ion therapy. Studies show that it has been possible to successfully treat with carbon ions, with a dose of 55Gy in 12# being tolerated by patients compared to stereotactic radiation therapy with photons reaching an average dose of 30-45Gy.
One of the first European studies into the use of carbon ions specifically to treat pancreatic cancer explored more closely gastrointestinal toxicity (14). They concluded that all participants were able to complete the course of treatment. Whilst nausea and diarrhoea were still experienced, it was tolerated and resolved again after treatment was completed.
Could upright radiotherapy revolutionize the treatment of pancreatic cancer?
There are a very limited number of facilities that house carbon ion units despite the clear clinical benefits of this treatment modality. The size and expense of these machines is a big factor in this. Carbon ion machines, particularly those with a rotating gantry are very large and the equipment very heavy. Rotating gantries are typically approximately 11 meters in diameter, 25 meters in length and around 600 tons in weight (15).
To reduce the cost of housing a carbon ion machine, some facilities install fixed-beam carbon ion units where the beam remains static rather than rotating around the patient, however these still require large builds. The Gunma University Hospital facility reported that the building for their fixed beam machine was 2 stories below ground and one above making it around 20 meters tall (16). With a fixed beam and the patient lying supine, there are limitations on which treatment angles are achievable and therefore not all patients and indications can be treated with this setup. Alternatively, treatment time is extensive due to multiple changes in patient setup in order to facilitate multiple beam angles (9).
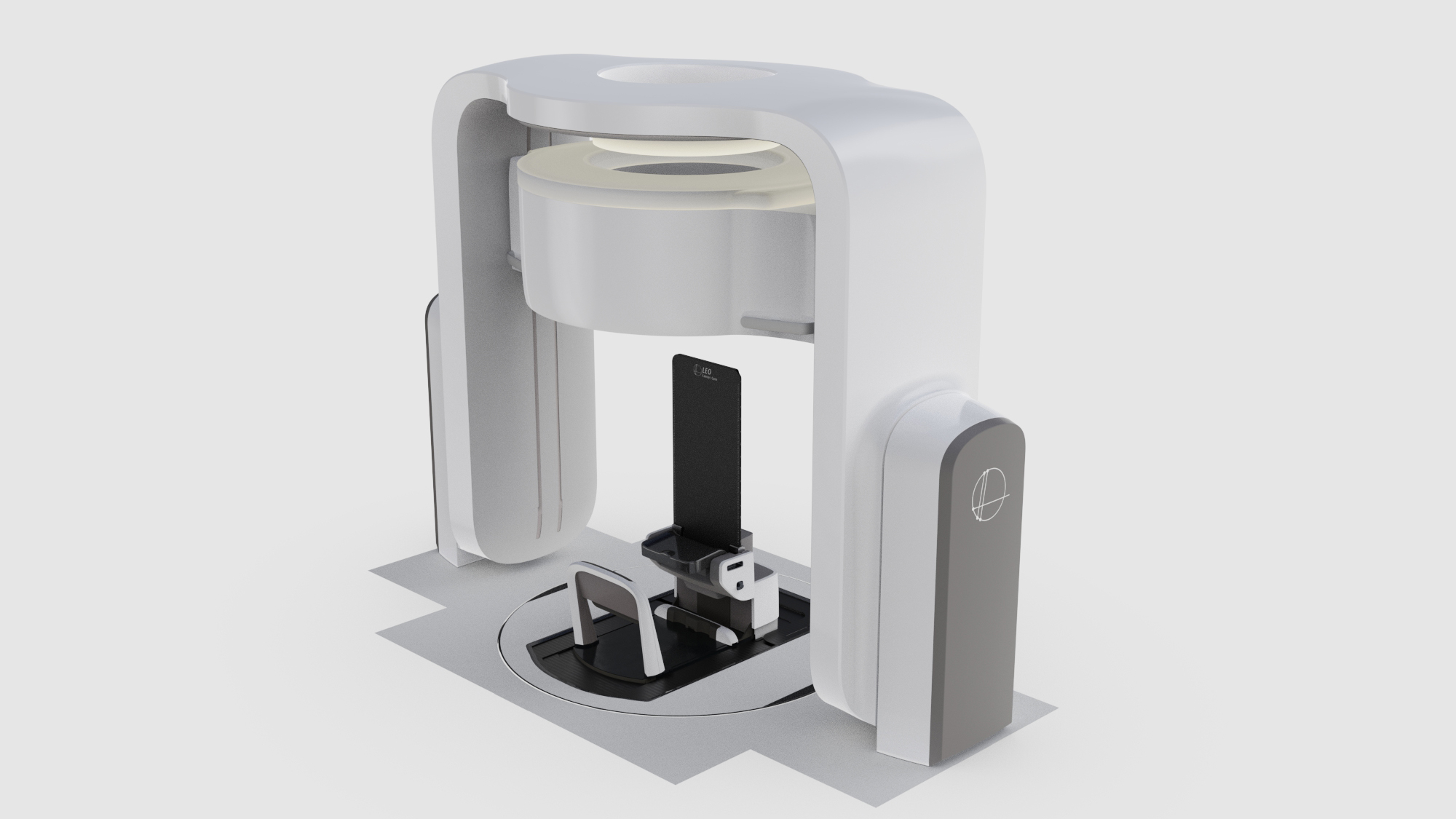
The optimal treatment angles for treating the pancreas include two posterior oblique fields to increase plan robustness against factors including undesirable doses to healthy tissue at the entry point, avoiding the high-density areas of the treatment couch and avoiding gastrointestinal organ filling in the beam pathway which can attenuate the beam affecting dose distribution conformity at the target (17). These posterior oblique fields are not achievable without a rotating gantry or an upright patient positioning system that rotates in front of a fixed beam source.
Currently, patients are frequently treated in a prone position to overcome planning constraints and to reduce the range of uncertainty caused by bowel gas and skin surface (9). However, if you are utilizing a fixed beam, ultimately the beam will cross the spinal cord. Also, it is not always a position that is comfortable for patients or one that is easy for patients to maneuver themselves into. Mobility limitations amongst some patients make it difficult to mount the supine treatment couch and assume this position, often requiring greater manual handling input from treatment radiographers, increasing their risk of musculoskeletal injuries (18). The prone position itself might not be comfortable for patients experiencing symptoms of their cancer which includes diarrhea, constipation, nausea, bloating, and pain at the top part of the stomach and back. A person experiencing nausea is advised to remain seated to avoid gastric fluids rising which can exacerbate nausea, they should also avoid crunching of the stomach which would be inevitable when maneuvering into a supine or prone treatment position (19).
By treating a patient in an upright position, patients can experience a more comfortable treatment position. By using an upright patient positioning system that rotates, it is possible to achieve the posterior oblique fields that are essential for tumor coverage and dose distribution with dose sparing to the spinal cord and surrounding tissue, this has historically limited dose escalation and impaired patient quality of life with associated toxicities.
Pancreatic cancer is subject to respiratory motion and research has found that in an upright orientation, breathing motion is reduced by up to 5mm (20). This is advantageous in treatment planning and delivery as treatment margins can be reduced due to reduced tumor motion. Current methods of managing respiratory motion include breath-holding and respiratory gating as well as image-guidance being an essential tool in planning, adapting and verifying treatment (15). Breath holding in an upright position is favorable for many patients with lung volumes increasing up to 50% when upright (20). A study by Boisbouvier et al (21) found that 53% of patients reported that breathing was ‘very easy’ upright compared to 40% saying the same when lying supine. Respiratory gating technology is currently being adapted to fit upright patient positioning systems currently available on the market and those under development with preliminary research from this suggesting that its use upright is feasible.
Computed tomography is still the current clinical standard for image guidance in radiation therapy. Upright computed tomography within a radiotherapy setting has been developed by Leo Cancer Care with the development of Marie™ coupled with the upright patient positioning system Eve®. Therefore, it is possible to image and verify in the treatment position using the industry standard imaging modality directly before treatment delivery supporting the implementation of adaptive radiotherapy which is essential in the treatment of pancreatic cancer (22).
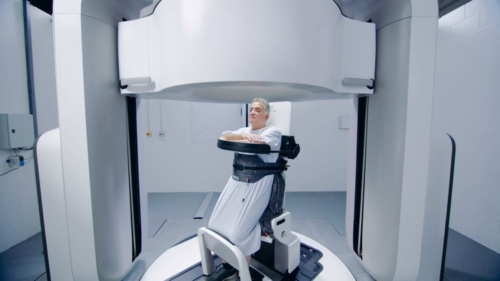
Patients have already shown a preference for an upright position. One study found that 87% of patients felt comfortable in the upright patient position compared to 73% when lying supine for treatment and 94% of patients said they found it easy to get in and out of the system compared to 60% saying the same about the supine treatment couch (21).
Upright patient positioning offers a cost-reducing strategy to increase global access to carbon ion therapy by supporting fixed-beam delivery while maintaining beam orientation and target dose distribution via patient rotation rather than radiation source rotation.
By coupling upright positioning with carbon ions it is possible to offer patients a treatment that is more comfortable for them as well as one that has clear clinical benefits and has the ability to positively influence the harsh statistics that surround this formidable disease.
Please note: Leo Cancer Care’s upright patient positioning system as well as the upright CT scanner have recently gained 510(k) regulatory clearance in the United States for clinical use.
References
- Pancreatic Cancer UK. Pancreatic Cancer Statistics. (2024). Available at: https://www.pancreaticcancer.org.uk/what-we-do/media-centre/pancreatic-cancer-statistics/ (Accessed: 22nd July 2024)
- Post, E. Five-year Pancreatic Cancer Survival Rate Increases to 12%. (2023). Available at: https://pancan.org/news/five-year-pancreatic-cancer-survival-rate-increases-to-12/#:~:text=Still%2C%20there’s%20more%20work%20to,from%20the%20disease%20this%20year. (accessed: 22nd July 2024)
- Zhang, L. et al. A population-based study of synchronous distant metastases and prognosis in patients with PDAC at initial diagnosis. Frontiers in Oncology. (2023) 13:1087700. doi: 10.3389/fonc.2023.1087700
- Falco, M., Masojc, B., Sulikowski, T. Radiotherapy in Pancreatic Cancer: To Whom, When, and How? Cancers. (2023) 15, 3382. doi: 10.3390/cancers15133382
- Liu, P., et al. Investigate the Dosimetric and Potential Clinical Benefits Utilizing Stereotactic Body Radiation Therapy with Simultaneous Integrated Boost Technique for Locally Advanced Pancreatic Cancer: A Comparison Between Photon and Proton Beam Therapy. Frontiers in Oncology. (2021) 11: 747532. doi: 10.3389/fonc.2021.747532
- Rutenberg, M., Nicholas, R. Proton beam radiotherapy for pancreas cancer. J Gastrointest Oncol. (2020) 11(1): 166-175. doi: 10.21037/jgo.2019.03.02
- Almog, G., et al. Pancreatic cancer outcome-local treatment with radiation using MRI-LINAC. Frontiers in Oncology. (2023) 13: 1289919. doi: 10.3389/fonc.2023.1289919
- Malouff, T., et al. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Frontiers in Oncology. (2020) 10:82. doi: 10.3389/fonc.2020.00082
- Koom, WS., et al. Beam direction arrangement using a superconducting rotating gantry in carbon ion treatment for pancreatic cancer. Br J Radiol. (2019) 92(1098): 20190101. doi: 10.1259/bjr.20190101
- Ishikawa, H., et al. Carbon-ion radiotherapy for urological cancers. International Journal of Urology. (2022) 29(10): 1109-1119. https://doi.org/10.1111/iju.14950
- Koosha, F., Ahmadikamalabadi, M., Mohammadi, M. Review of Recent Improvements in Carbon Ion Radiation Therapy in the Treatment of Glioblastoma. Advances in Radiation Oncology. (2024) 9(5): 101465. DOI:https://doi.org/10.1016/j.adro.2024.101465
- Shinoto M, Yamada S, Terashima K, Yasuda S, Shioyama Y, Honda H, et al. Carbon Ion Radiation Therapy With Concurrent Gemcitabine for Patients With Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys. (2016) 95(1):498–504. doi: 10.1016/j.ijrobp.2015.12.362
- Kawashiro S, Yamada S, Okamoto M, Ohno T, Nakano T, Shinoto M, et al. Multi-Institutional Study of Carbon-Ion Radiotherapy for Locally Advanced Pancreatic Cancer: Japan Carbon-Ion Radiation Oncology Study Group (J-CROS) Study 1403 Pancreas. Int J Radiat Oncol Biol Phys (2018) 101(5):1212–21. doi: 10.1016/j.ijrobp.2018.04.057
- Liermann, J., et al. Effectiveness of Carbon Ion radiation in locally advanced pancreatic cancer. Frontiers in Oncology. (2021) 10 (82) 1-13 doi. 10.3389/fonc.2021.708884
- Meschini, G., et al. Time-resolved MRI for off-line treatment robustness evaluation in carbon-ion radiotherapy of pancreatic cancer. Medical Physics. (2022) 49(4): 2386-2395. doi: 10.1002/mp.15510
- Ohno, T., et al. Carbon Ion Radiotherapy at the Gunma University Heavy Ion Medical Center: New Facility Set-up. Cancers (Basel). (2011) 3(4): 4046-4060. doi: 10.3390/cancers3044046
- Kawashima, M., et al. An adaptive planning strategy in carbon ion therapy of pancreatic cancer involving beam angle selection. Physics and Imaging in Radiation Oncology. (2022) 21:35-41. doi: 10.1016/j.phro.2022.01.005
- Society of Radiographers. Musculoskeletal Disorders in Therapeutic Radiographers. (2012). Available at: https://www.sor.org/learning/document-library/musculoskeletal-disorders-therapeutic-radiographers
- McDermott, A. Top 18 ways to get rid of nausea. (2024) Available at: https://www.healthline.com/health/how-to-get-rid-of-nausea#:~:text=Sit%20up%20and%20avoid%20crunching%20the%20stomach&text=When%20you%20lie%20flat%2C%20gastric,you%20less%20comfortable%20in%20general. (Accessed 1/8/2024)
- Yang, j., et al. Advantages of simulating thoracic cancer patients in an upright position. Practical Radiation Oncology. (2024) 4(1):e53-8 doi: 10.1016/j.prro.2013.04.005. Epub 2013 Jun 14.
- Boisbouvier, S. et al. Upright patient positioning for pelvic radiotherapy treatments. Technical Innovations & Patient Support in radiotherapy treatments. (2022) 24: 124-130. doi: http://doi.org/10.1016/j.tipsro.2022.11.003
- Bhattachryya, T., et al. First prospective feasibility study of carbon-ion radiotherapy using compact superconducting rotating gantry. British Journal of Radiology. (2019) 92 (1103): 20190370. doi: 10.1259/bjr.20190370
- Tinganelli, W., Durante, M. Carbon Ion Radiobiology. Cancers (Basel). (2020) 12(10): 3022. doi: 10.3390/cancers12103022
Subscribe to our newsletter
STAY CONNECTED
Get the latest updates and exclusive offers delivered straight to your inbox
Subscribe